Stochastic electrotransport selectively enhances the transport of highly electromobile molecules [full article]
Sung-Yon Kim, Jae Hun Cho, Evan Murray, Naveed Bakh, Heejin Choi, Kimberly Ohn, Sara Vassallo, Luzdary Ruelas, Austin Hubbert, Meg McCue, Philipp Keller and Kwanghun Chung. Stochastic electrotransport selectively enhances the transport of highly electromobile molecules, PNAS, 2015 Nov 17: 112(46): E6274-83. doi: 10.1073/pnas.1510133112. Epub 2015 Nov 2. PubMed PMID: 26578787; PubMed Central PMCID: PMC4655572.
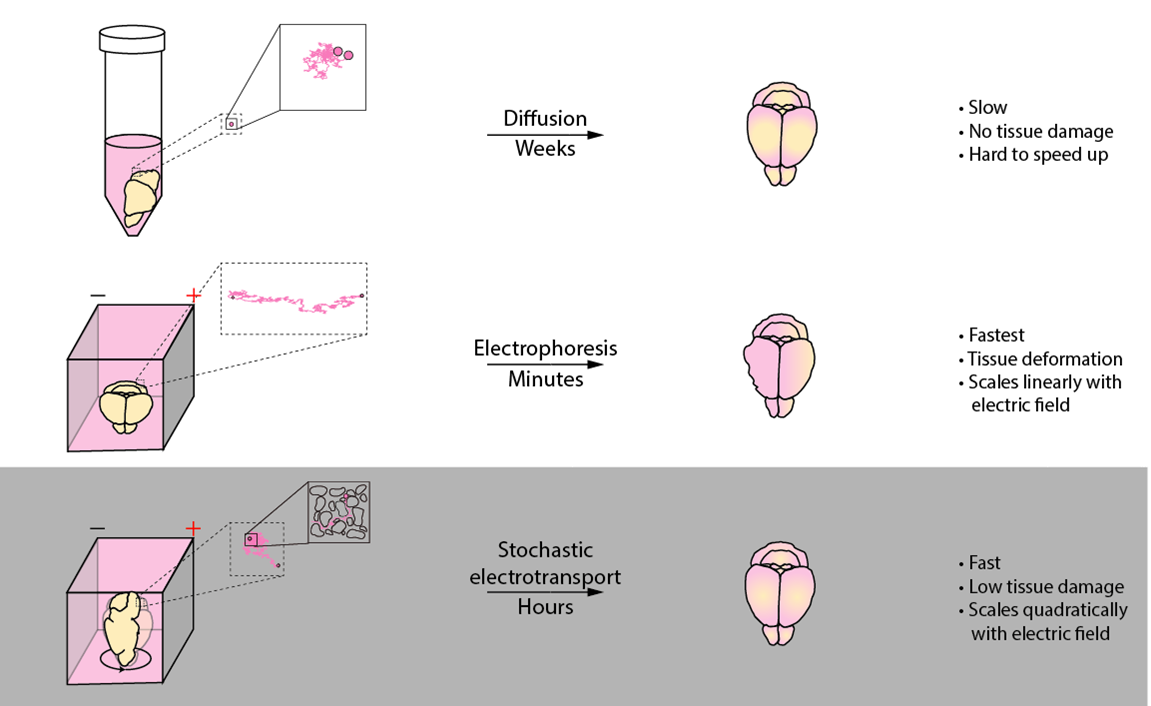
Stochastic electrotransport is a transport enhancement method designed specifically for biological tissues. Like electrophoresis, it uses electric fields to drive electromobile molecules into the tissue. But unlike electrophoresis, it does not cause damage to the tissue. This allows you to quickly process tissues without destroying them.